The concentration of H3O+ in the solution is 6.667*10^-11M.
1st) From the dissociation equation of NaOH we calculate the concentration of OH- in the 0.0150M solution:
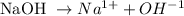
According to the equation, from 1 mol of NaOH we obtain 1 mol of OH-, so from the 0.0150M solution, we will obtain a 0.0150M concentration of OH-.
2nd) Using the Kw formula and replacing the values, we can calculate the H3O+ concentracion:
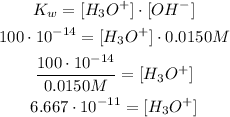
So, the concentration of H3O+ in the solution is 6.667*10^-11M.