GIven:
The diameter of the inside portion of the given cylinder is 28cm.
The height of the cylinder is 46cm.
The density of Carbon dioxide is 1.9359 kg/ cubic meters.
The density of Helium is 0.1761 kg/ cubic meters.
The density of Chlorine is 3.1205 kg/ cubic meters.
The cylinder is filled with one of these gases.
Required:
We need to find the heaviest weight.
Step-by-step explanation:
Consider the radius formula.
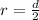
Substitute d =28 in the formula.
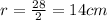
Consider the volume of the cylinder formula.
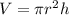
Substitute r =14 and h =46 in the formula.
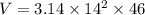
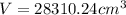
We know that
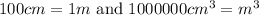
Multiply the volume by 1000000 to convert cm to m.
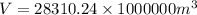
We know that
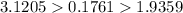
Chlorine has the heaviest density of the given three gases. So Choline gives the heaviest weight since density is directly proportional to the weight.
Multiply the volume by the density of the Chlorine to find the weight of the Chlorine.
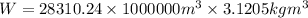
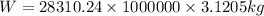
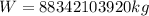
Final answer:
The heaviest weight of the given three gases is 88342103920 kg.