Answer:
7 m³
Step-by-step explanation:
At a constant temperature, we have the following relationship between volume and pressure:
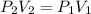
Where P is the pressure and V is the volume. So, we can solve for the final volume V2 as:
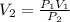
Replacing P1 = 4 atm, V1 = 10.5 m³, and P2 = 6 atm, we get:
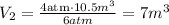
Therefore, the final volume of the lungs will be 7 m³