Answer:
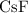
Step-by-step explanation:
Here, we want to get the most ionic fluoride
Cs is a group 1 element, same as sodium
However, we know that electropositivity increases down the group while electronegativity decreases down the group
Also, electropositivity decreases across the period. It is expected that a group 1 element will be more electropositive than a group 3 element.
Thus, we are having a bond between a very electropositive metal and a very electronegative non-metal. That is why we have CsF (it is the strongest and most electropositive atom in the options listed) as the molecule with the strongest ionic bond on the list