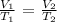Answer:
Step-by-step explanation:
Here, we want to describe the relationship between the volume and temperature of an ideal gas
This relationship is defined by Charles' law
From this law, we know that the volume of a given mass of gas is directly proportional to its temperature at a fixed pressure
What this means is that as long as the pressure remains unchanged, when the volume increases, the temperature increases, and when the volume decreases, the temperature decreases
These can be represented by the mathematical formula below: