INFORMATION:
We know that:
- Sodium azide, NaN3, decomposes according to the following equation 2NaN3(s) → 2Na(s) + 3N2(g)
- We have an 45.0L airbag at 25°C and a pressure of 809 torr
And we must find how many grams of sodium azide are needed to inflate the airbag
STEP BY STEP EXPLANATION:
To find it, we need to use the Ideal Gas Law formula PV = nRT
Where,
P = pressure
V = volume
n = number of moles
T = temperature
R = gas constant
Is given that;
P = 809 torr
V = 45.0L
n = we need to calculate it
T = 25°C, converting to K, 25 °C + 273.15 = 298.15 K
R = 62.36 (L*torr)/(mol*K)
Now, replacing in the formula,
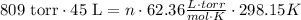
Now, solving for n
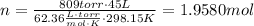
Now, using that molar mass of NaN3 = 65.02 g/mol, we can calculate the grams
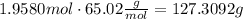
Then, 127.3092 g are needed to inflate the airbag.
ANSWER:
127.3092 grams are needed to inflate the airbag.