Step 1 - Converting mol/L to g/L
To convert moles to grams we just need to multiply the number of moles by the molar mass of the substance. We can do the exact same process to convert a unity concentration (mol/L) into another unity concentration (g/L).
Since we already know the molarity (mol/L) of acetic acid (0.8393 mol/L) and we know its molar mass (60,052 g/mol), we can find its concentration in g/L:
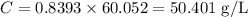
Step 2 - Finding out the mass of acetic acid in 1 ml of solution
As we have calculated in the previous step, there are 50.041 g of acetic acid per liter of solution. Since 1 L corresponds to 1000 ml, we can use it to calculate the amount of acetic acid in 1 ml of solution:
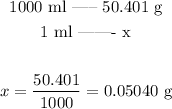
This is the mass of acetic acid in 1 ml of solution
Step 3 - Using the density to discover the percentage of acetic acid
Since we know the density of the solution (1.006 g/cm3), we know that the mass of acetic acid plus water in 1 ml (1 cm3) corresponds to 1.006. This is the total mass in 1 ml.
As we discovered in the previous step, the mass of acetic acid in 1 ml is 0.05040 g. We just need to discover what percentage this value represents in relation to the total mass:
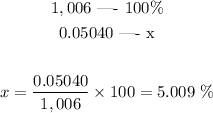
The mass percentage of acetic acid is thus 5.009 %.