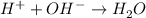Step-by-step explanation:
Firstly, we need to define a base and alkali
A base is a substance that can neutralize the acid by reacting with hydrogen or hydroxonium ions
An alkali is a soluble base. This implies that alkali is a base that dissolves in water
Hence, all Akali are bases and all bases are not alkali
When the base neutralizes acid by reacting with a hydrogen ion, the resulting ions will be negatively charged which is the Hydroxyl ion