Answer
b. 95%
Step-by-step explanation
Given:
Mass of K₂O produced (actual yield) = 28.56 g
Mass of K that reacted = 25.00 g
Equation: 4K(s) + O₂(g) → 2K₂0(s)
What to find:
The percent yield of K₂O.
Step-by-step solution:
The first step is to calculate the theoretical yield of K₂O produced.
From the balanced equation, 4 mol K produced 2 mol K₂O
Molar mass of K₂O = 94.20 g/mol)
Molar mass of K = 39.10 g/mol)
This means 4 mol x 39.10 g/mol = 156.40 g K produced 2 mol x 94.20 g/mol = 188.40 g K₂O
So 25.00 g K will produce:
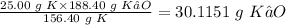
Actual yield of K₂O = 28.56 g
Theoretical yield of k₂O = 30.1151 g
The percent yield for the reaction can now be calculated using the formula below:
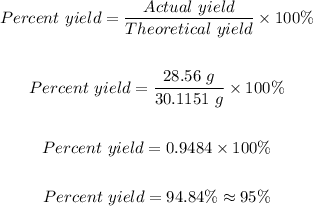
Therefore, the percent yield for the reaction is 95%.