ANSWER
The volume of hydrogen gas is 62.16L
Step-by-step explanation
Given information
The mass of lithium metal = 38.5 grams
The first step is to write a balanced chemical reaction equation for the reaction
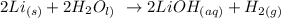
From the above reaction, you can see that 2 moles of lithium metal react with 2 moles of water to give 2 moles of lithium hydroxide and 1 mole of hydrogen gas
The next step is to find the mole of lithium metal using the below formula
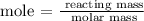
According to the periodic table, the molar mass of lithium metal is 6.941 u
Recall that, the mass of lithium is 38.5 g
Therefore, the mole can be calculated as
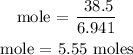
From the above calculation, you will see that the mole of lithium metal is 5.55 moles
The next step is to find the mole of hydrogen gas using a stoichiometry ratio
From the reaction above, you will see that 2 moles of lithium metal give 1 mole of hydrogen gas
Let x represents the number of moles of hydrogen gas
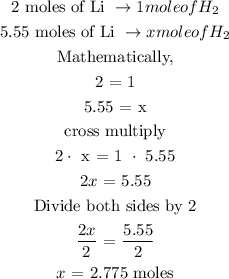
Therefore, the number of moles of hydrogen gas is 2.775 moles
The next step is to find the volume of hydrogen gas from the calculated number of moles
Recall that at STP, 1 mole is equivalent to 22.4 L
Let x represents the volume of the hydrogen gas
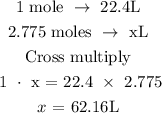
Therefore, the volume of hydrogen gas is 62.16L