Answer:
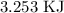
Step-by-step explanation:
Here, we want to calculate the quantity of heat in KJ
Mathematically, we can calculate that as follows:
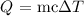
where:
m is the mass of the aluminum piece which is 79.5 g
c is the specific heat capacity of the aluminum which is 0.930 J/g.°C
deLta T is the change in temperature which is the difference in temperature of the final and initial temperature
Substituting the values, we have it that:
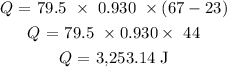
To convert this to KJ, we have to divide by 1000
Thus, we have the value in KJ as 3.253 KJ