1) Write the atom-molecule ratio.
1 Fe: 1FeCl3
2) Moles of FeCl3 produced from Fe. Assuming that Fe is the limiting reactant.
2.1- Convert grams of Fe to moles of Fe.
The molar mass of Fe is 55.8450 g/mol
Sample: 311 g Fe
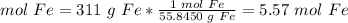
We have 5.57 mol Fe
2.2- FeCl3 produced.
5.57 mol Fe
1 Fe: 1FeCl3
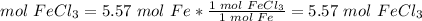
5.57 mol FeCl3 would be produced from 311 g Fe. Assuming that Fe is the limiting reactant.
.