Answer:
200.98grams
Explanations:
The balanced chemical reaction between Lithium and nitrogen is given as:
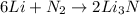
Given the following parameter
Mass of Li3N = 500grams
Determine the moles of Li3N
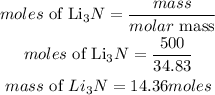
Based on stoichiometry, 1 mole of nitrogen produced 2 moles lithium nitride. The moles of nitrogen required will be:
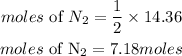
Determine the mass of nitrogen that reacted
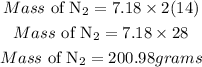
Hence the mass of nitrogen that has reacted if 500g of Li3N was produced is 200.98grams