Answer:
3,961J are needed. (The closest option is B. +3963J).
Step-by-step explanation:
The given information from the exercise is:
- Mass of water: 10.0g
- Initial temperature of ice: -10°C
- Final temperature of water: 10°C
To calculate the heat of each parte of the melting process, it is also necessary to use the following information:
- Heat of fusion (hf): 334 J/g
- Specific heat capacity of ice (ci): 2.03 J/g.°C
- Specific heat capacity of water (cw): 4.18 J/g.°C
To calculate the total heat of the melting process, it is necessary to calculate 2 types of heat:
• (Q1) Heat needed to raise the temperature of ice from -10°C to 0°C:
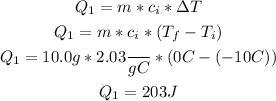
• (Q2) Heat required to transform the entire mass of ice into water:
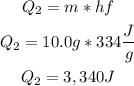
• (Q3) Heat needed to raise the temperature of water from 0°C to 10°C:
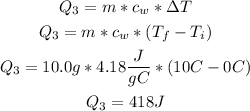
Finally, we have to add each heat value (of each part of the process), to calculate the total heat needed:
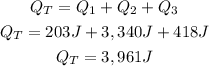
So, 3,961J are needed. (The closest option is B. +3963J).