Answer:
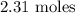
Step-by-step explanation:
Here, we want to get the number of moles of sulfur dioxide needed to form 2.31 moles of sulfur trioxide
The first thing we would be needing here is to write the equation of the reaction
We have that as:
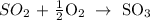
From the equation of reaction, the number of moles are in the same ratio
1 mole of sulfur dioxide formed 1 mole of sulfur trioxide
That means:
2.31 moles of sulfur trioxide was formed from 2.31 moles of sulfur dioxide