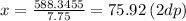Answer:
75.92
Step-by-step explanation:
A)the % abundance adds up to 100, so adding all the % abundances of Ge:
20.52 + 27.45 + 7.76 + 36.52 = 92.25
The remaining % abundance is:
100 - 92.25 = 7.75%
B) Using this formula:
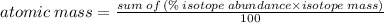
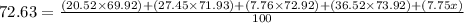
multiply both sides by 100 to get rid of the fraction:
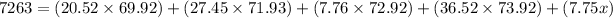
multiplying each bracket:
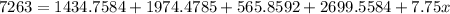
adding up the terms:
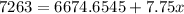
subtract 6674.6545 on both sides to give:
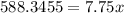
divide both sides by 7.75: