Answer:
7.835 moles of H2O.
Step-by-step explanation:
What is given?
Particles of H2O = 4.718 x 10²⁴ particles.
Avogadro's number = 6.022 x 10²³ particles /mol.
Step-by-step solution:
To find the moles in a certain number of particles, we have to use Avogadro's number which is 6.022 x 10²³ particles/mol. This is telling us that there are 6.022 x 10²³ particles of a certain compound in 1 mol. The conversion from particles to moles will look like this:
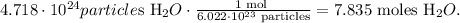
The answer is that there are 7.835 moles of H2O in 4.718 x 10²⁴ particles of H2O.