Potassium nitrate has the formula KNO₃. We can calculate the molar mass from the atomic masses of the element in KNO₃. We have 1 K, 1 N and 3 O, so:
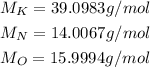
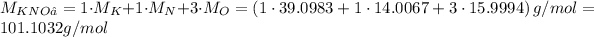
Since we want a 0.700 M solution with volume of 0.250 L, we can calculate the number of moles of KNO₃ needed:
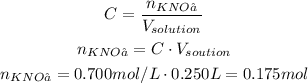
Now, we can convert the number of moles to mass using the molar mass, M:
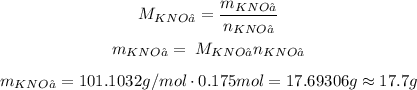
So, we would need approximately 17.7g.