ANSWER
The mass of iron is 296.70 grams
Explanation:
Given information
Mass of sulfur = 170 g
Let x represents the mass of iron
The next step is to write a balanced chemical equation for the reaction
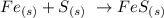
From the balanced chemical equation above, you will see that one mole of sulfur reacts with one mole of iron to produce one mole of iron sulfide
The next thing is to find the mole of sulfur using the below formula
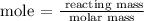
According to the periodic table, the molar mass of sulfur is 32 g/mol
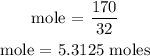
To find the mole of iron, we will be using a stoichiometry ratio

Therefore, the moles of iron is 5.3125 moles
The next step is to find the mass of iron using the below formula
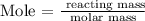
From the periodic table, the molar mass of iron is 55.85g/mol
Let x be the mass of iron
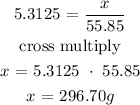
Therefore, the mass of iron is 296.70 grams