Givens.
• 20 L of Oxygen.
,
• 5 volume O2 = 4 volume of H2O.
Remember that volume stoichiometry allows us to use the coefficients as volume.
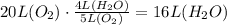
(1) Therefore, 20 L of oxygen will produce 16 L of water vapor.
Keep using volume stoichiometry (coefficients) to form ratios and find the volumes.
• 5 volume O2 = 3 volume CO2.
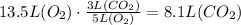
(2) Therefore, 13.5 L of oxygen will make 8.1 L of CO2.