Given:
Volume of canister, V1 = 1500 ml
Temperature, T1 = 22°C
Final temperature, T2 = 0°C
Let's find the volume the gas will occupy if the pressure remains constant.
To find the volume, V2, if the pressure remains constant, apply Charles' law:
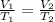
Where:
V1 = 1500 ml
T1 = 22°C
T2 = 0°C
Let's solve for V2.
First convert the temperature from degrees celsius to Kelvin.
Where:
0°C = 273K
T1 = 22°C + 273 = 295K
Hence, we have:
T1 = 295K
T2 = 273K
Thus, solving for V2, 23 have:
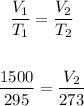
Cross multiply:
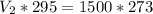
Divide both sides by 295:
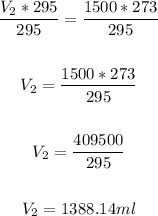
Therefore, the volume the gas will occupy if the pressure remains constant is 1388.14 ml.
ANSWER:
1388.14 mL