Answer:
159.77kJ
Explanations:
Given the balanced chemical reaction between carbon C(s) and SO2(g) expressed as:
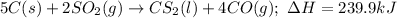
First, we need to get the number of moles of Carbon present using the formula;
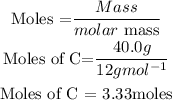
According to the reaction, 5 moles of carbon reacts to produce 239.9 kJ of heat.
To determine how much heat will be produced by 3.33 moles of Carbon, this will be expressed as:
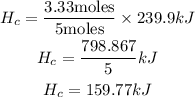
Hence the amount of heat absorbed when 40.00 g of C( s) reacts in the presence of excess SO 2( g) to produce CS 2( l) and CO(g) is 159.77kJ