ANSWER
The volume of fluorine is 510.52L
Explanation:
From the question provided, you were given the mass of fluorine to be 432.8 grams.
Given information
Mass of fluorine = 432.8 grams
To find the volume of Fluorine, we need to find the mole of fluorine from the mole formula
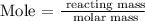
From the periodic table, the molar mass is 18.99 g/mol
The next thing is to substitute the data into the formula
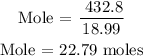
Since the mole of fluorine is 22.79 moles, then, we can now find the volume of fluorine.
At STP, 1 mole is equivalent to 22.4L
Let the volume of fluorine be x
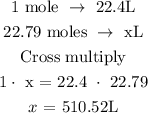
The volume of fluorine is 510.52L