If the atom is neutral, it means that there are the same number of electrons and protons. Then, the number of electrons of the atom is 2.4*10^-12 C.
The number of electrons is obtained by using the following equivalence:
1C = 6.24*10^18 e
Then, you have:
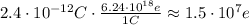
Hence, there are approximately 1.5*10^7 electrons in the neutral atom