As the ionisation energy is the amount of energy required to remove the outermost electron from an atom.
With the increase in the atomic number, the number of shells are increasing. Due to which the distance between the outer electron and the nucleus is also increasing.
According to the coulomb's law,
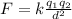
where F is the force of attraction between the two charges.
In the case of atom,
Let F be the force of attraction between the outer most electron and nucleus.
d is the distance between the nucleus and outermost electron.
As the atomic number increases from helium to radon, the distance between the nucleus and the outermost electron is also increasing.
Thus, the force of attraction between the nucleus and electron decreases.
Hence, the ionisation energy required to remove the outermost electron from helium to radon is decreasing.