ANSWER
The final pressure of the gas is 1001.6 mmHg
Step-by-step explanation
Given that;
The pressure of the gas is 750 mmHg
The temperature of the gas is 25 degrees celcius
The final temperature of the gas is 125 degrees Celcius
Follow the steps below to find the final pressure of the gas
In the given data, the volume of the gas is fixed, hence, we can apply Gay Lussac's law to find the final pressure of the gas
Step 1; States the Gay Lussac's law
Gay Lussac's a gas law which states that the pressure exerted by a gas (of a given mass and kept at a constant volume) varies directly with the absolute temperature of the gas.
Mathematically, this can be expressed as
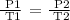
Step 2: Convert the temperature to degrees Kelvin
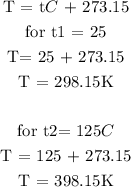
Step 3: Substitute the given data into the formula in step 1
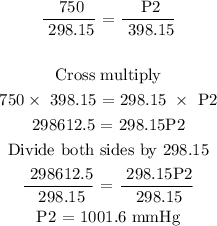
Therefore, the final pressure of the gas is 1001.6 mmHg