Answer:
The setup calculation shown considers a gas law (Charle's Law), which must be used with the absolute scale of temperature. However, Carmen did not use the absolute scale, which can explain why the results obtained by the two students were different.
Step-by-step explanation:
The question requires us to analyze the setup for a gas law calculation and identify possible mistakes in it.
The setup shown for the gas law calculation considers volume (V) and temperature (T) at constant pressure, which can be described mathematically as:
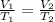
The gas law represented by the question, known as Charle's Law, considers an isobaric process (i.e., that occurs at constant pressure, and tells us that volume and temperature are directly proportional under this condition.
An important detail when applying the Charle's Law (as well as other gas laws), is that the temperature must be considered at the absolute scale (in other words, in units of Kelvin, K). When applying the temperature in a different scale, such as in Celsius degrees, the result obtained will not be correct.
We can see in the image that the calculation set up by Carmen was done using Celsius degrees, which can explain why the two students mentioned obtained different answers.