The absolute entropy of copper at 20K can be calculated by integrating the molar heat capacity over the temperature range from 0K to 20K and dividing by the temperature.
The formula is:

Using the given equation for Cp, we get:
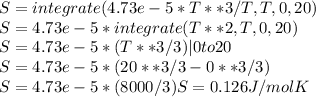
Therefore, rounding up the absolute entropy of copper at 20K is 0.13 J/molK.