ANSWER
Step-by-step explanation
Given that;
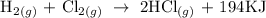
The above reaction is an example of an exothermic reaction.
Recall, that exothermic reaction is a type of reaction in which heat is liberated to the surroundings during the reaction.
To indicate the shift of the equilibrium on the reaction, apply Le Chatelier's principle
Le Chatelier's principle states that when an external constraints such as concentration, temperature or pressure is imposed on a system in equilirium, the equilibrium shift so as to annul the effect the of the constraints.
Since Cl2 is one of the reactants, thtion, tempe