ANSWER
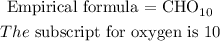
Step-by-step explanation:
Given information
The moles of carbon = 1.3 moles
The moles of hydrogen = 1.3 moles
The moles of oxygen = 13.0 moles
From the given data above, it is easy to determine the empirical formula of the compound
Since the moles of each element is given in the question provided, then we can now find the empirical formula by finding the mole ratio of the elements
To find the mole ratio, we will need to divide the moles by the smallest number of moles.
Therefore, the empirical formula of the compound is given below as
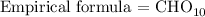
From the empirical formula above, the subscript for oxygen is 10