Answer:
28.22L of HF are needed.
Step-by-step explanation:
1st) It is necessary to convert the 14.2L H2 to moles, using the relation for gases that 22.4L is equal to the volume of 1 mole:
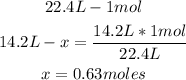
2nd) Now, with the stoichiometry of the balanced reaction (we know that 1 mole of H2 is formed from 2 moles of HF), and the 0.63 moles of H2, we can calculate the moles of HF needed:
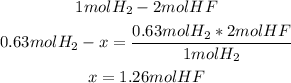
Now we know that 1.26 moles of HF can produce 14.2L (0.63moles) of H2 gas.
3rd) Finally, we have to convert the 1.26 moles of HF to liters:
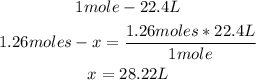
So, 28.22L of HF are needed.