According to the chemical equation, the reaction absorbs 54.8 kJ per 2 moles of NH4Cl because that's the stoichiometry coefficient of this molecule.
Now, we need to find the number of moles in each mole of NH4Cl.
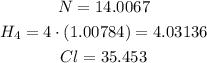
Then, we add them to find the molar mass.
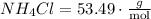
This means that 1 mole of NH4Cl equals 53.49 grams.
At last, we multiply the mass of NH4Cl (27.1 g) by two ratios, the first ratio is about the molar mass, and the second ratio is about the amount of heat per moles.
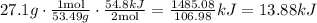
Therefore, the amount of energy that would be absorbed is 13.88 kJ.