ANSWER
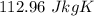
Step-by-step explanation
Parameters given:
Mass of sample, m = 0.5 kg
Temperature change, ΔT = 5.40 °C = 5.40 K
Heat energy, H = 305 J
To find the specific heat capacity of the sample, we have to apply the formula for heat energy:
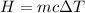
Where c = specific heat capacity
Therefore, solving for c, the specific heat capacity of the metal sample is:
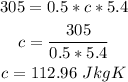
That is the answer.