Answer
The partial pressure of nitrogen = 579.54 mmHg.
Step-by-step explanation
Given that nitrogen makes up about 78 % of atmospheric air.
Mole fraction of nitrogen in the atmosphere = 78/100 = 0.78
Total pressure of the air = 743 mmHg
So according to Raoult's law:
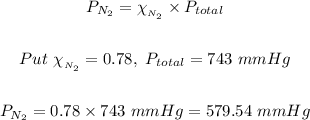
The partial pressure of nitrogen = 579.54 mmHg.