ANSWER
Step-by-step explanation
Given that
The energy released by the system is 12.4J
Work done on the surrounding is 4.2J
Follow the steps below to find the change in energy
In the given data, energy is said to be released to the surroundings
Recall, that exothermic reaction is a type of reaction in which heat is released to the surroundings. Hence, change in enthalpy is negative
Step 1; Write the formula for calculating change in energy
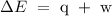
Since heat is released to the surrounding, then q = -12J
Recall, that work done by the system on the surroundings is always negative
Hence, w = -4.2J
Step 2; Substitute the given data into the formula in step 1
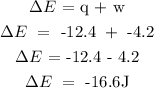
Therefore, the change i