The balanced equation of the reaction that they describe to us says:
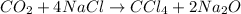
We see that 4 moles of NaCl produce 1 mole of CCl4. Therefore, the CCl4 to NaCl ratio is 1/4. So if we have 1.2 moles of NaCl, the moles of CCl4 that can be produced will be:
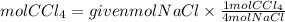
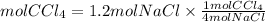
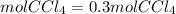
Answer: If we start with 1.2 moles of NaCl, it would be produced 0.3 moles of CCl4