Step-by-step explanation:
2H2O2 → 2H2O + O2
Oxidation numbers
On the left:
H2O2 => O (-1) and H (+1)
-----------
On the right:
H2O => O (-2) and H (+1)
O2 => 0
----------------------
REDOX reaction: the half-reactions
Oxidation:
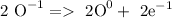
Reduction:
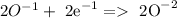
According to this, H2O2 is the oxidizind and the reducing agent.