Answer:
0.791grams
Explanations:
At standard temperature and pressure;
1 mole of gas = 22.4L
The number of moles of 0.250 L of chlorine gas will be expressed as:
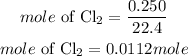
Determine the required mass
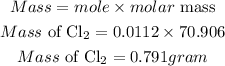
Therefore at STP, 0.250 L of chlorine gas will have a mass of 0.791grams