Given:
A chemist needs to make 3 mL of a 10% methane solution from a 6% methane solution and a 40% methane solution
Aim:
We need to find equations to find the number of ml of each solution.
Step-by-step explanation:
Let x be the number of mL of the 6% methane solution and y be the number of mL of the 40% methane
We know that the total number of ml of the 10% solution is 3ml.
The number of mL of the 6% methane solution + the number of mL of the 40% methane = the total number of ml of the 10% solution
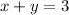
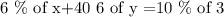
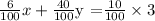
Multiply both sides by 100.
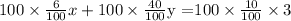
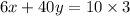
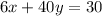
The required two equations are
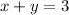
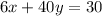
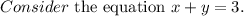
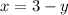
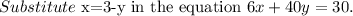
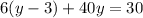
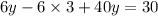
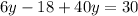
Solve for y.
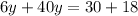
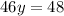
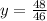
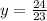
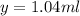
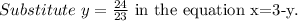
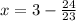
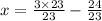
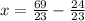
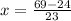
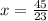
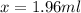
Final answer:
The equations:
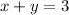
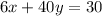
The number of mL of the 6% methane solution is 1.04 ml.
The number of mL of the 40% methane solution is 1.96 ml.