Answer:
Reaction type - Decomposition reaction
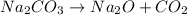
Explanations:
According to the question, we need to determine the type of reaction that occur.
The type of raction that will occur is a decomposition reaction. This ois the type of reaction that occur when the reactant breaks down into two or more product.
The decomposition of sodium carbonate will produce sodium oxide and carbon dioxide as shown:
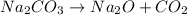
Hence the reation type is a decomposition reaction