Explanation:
Given parameters
The mass of CO2 = 44.6 grams
To find the volume, we need to find the mole of CO2 first
The formula for calculating mole is given below as
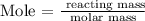
The molar mass of CO2 can be calculated using the formula unit
Mass of carbon = 12 u
Mass of oxygen = 16 u
Molar mass of CO2 = 12 + 2(16)
Molar mass of CO2 = 12 + 32
Molar mass of CO2 = 44 g/mol
Substituting the above parameters into the mole formula