Required: A general explanation on how to do solubility calculations
Answer
- What is solubility?
Solubility is a ratio that relates the maximum amount of a solute that can dissolve to a standardized 100 g or 100 mL of the solvent. What you do is divide the mass of the compound by the mass of the solvent and then multiply by 100 g to calculate the solubility in g/100g.
When calculating solubility, it is when you want to know if the solution is saturated or unsaturated, some solutions can be supersaturated.
For example:
Lets say you are given a solution that was prepared by mixing 129 grams of ammonium iodide and 75.0 grams of water at 20 C The first thing to do is to determine what is a solute, and which one is the solvent. In this case ammonium iodide is the solute and water is the solvent.
-To calculate the solubility of ammonium iodide in water:
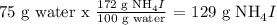
Therefore exactly 129 grams of ammonium iodide dissolves in 75 g of water. The resultant solution is saturated.
If the amount of solvent used to dissolve the solute is more, the solution is said to be supersaturated. And if the solute is more than the solvent it can dissolve, the solution is said to be unsaturated.