Step-by-step explanation
Given:
Mass of Li2SO4 = 400 g
Required: Mass of Pb(SO4)2
Solution
Step 1: Balance the equation
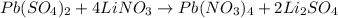
Step 2: Calculate the number of moles of Li2SO4
n = m/M where n is the moles, m is the mass and M is the molar mass (109,94 g/mol)
n = 400g/109,94 g/mol
n = 3.64 mol
Step 3: Use the stoichiometry to find the moles of Pb(SO4)2
The molar ratio between Pb(SO4)2 and Li2SO4 is 1:2
Therefore the moles of Pb(SO4)2 = 3.64 x (1/2) = 1.82 mol
Step 4: Calculate the mass of Pb(SO4)2
m = n x M
m = 1.82 mol x 303,26 g/mol
m = 551.93 g
Answer
Mass of Pb(SO4)2 = 551.93 g