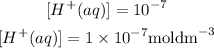Answer
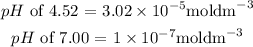
Explanations:
To calculate the pH of an aqueous solution, you need to know the concentration of the hydronium ion in moles per liter (molarity). The pH is therefore calculated using the formula;
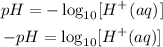
Note that the square bracket denotes concentration.
Recall from the law of logarithm;
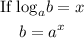
Applying this rule to the pH formula above, we will have:
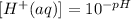
For the concentration with a pH of 4.52, its concentration will be derived by substituting this pH value into the concentration formula as shown:
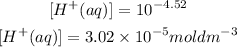
Similarly for the concentration of pH of 7.00;