We have to find the mass of a block of iron whose dimensions are 2.56cm×11.44cm×2.0cm, knowing its density: 7.86g/cm³.
For doing so, we remember that:
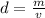
where d represents density, m mass, and v the volume. As we have the density, and we want to find the mass, we will find the volume of the block of iron, which has a shape of a prism.
Then, as the volume of a prism is:
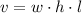
where w is width, h height, and l length, we have:
![\begin{gathered} v=2.56\operatorname{cm}\cdot11.44\operatorname{cm}\cdot2.0\operatorname{cm} \\ =58.5728\operatorname{cm}^3 \end{gathered}]()
Where the result has 4 significant figures as 2.56 has 2 (the 5 and 6), and 11.44 has 2 (the 4 and the 4).
For finding the mass, we will use the first formula, as we now have the volume and the density:
![\begin{gathered} d=(m)/(v) \\ d\cdot v=m \\ (7.86\frac{g}{\operatorname{cm}^3})(58.5728cm^3)=m \\ 460.382208g=m \end{gathered}]()
This means that the mass of the iron block is 460.382208 g.