To solve this problem, we have to find the volume of 1.00M HCl solution we have to add.
To do it, we have to use the formula for dilutions:
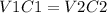
Where V1 is the initial volume, in this case it is unknown; C1 is the initial concentration that in this case is 1.00M; V2 is the final volume, that is 50.0mL and C2 is the final concentration that is 0.200M.
Solve the equation for V1 and replace for the given values:
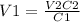
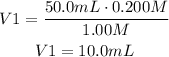
It means that we have to add 10.0mL of 1.00M HCl.
To find the volume of water, substract the initial volume of HCl to the final volume:
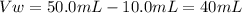
It means that the 10.0mL of HCl have to be added to 40mL of water.