Answer:
The ΔG° is 171,144.6 J/mol.
The reaction is not spontaneous.
Step-by-step explanation:
Given values:
T=25°C (298K)
Kp at 25°C= 1x10^-30
To calculate the ΔG° it is necessary to replace the value of R (8.314J/mol.K), temperature and Kp:
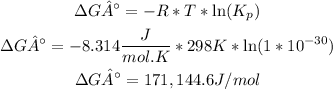
So, the ΔG° is 171,144.6 J/mol. Since ΔG° is greater than 0, the reaction is not spontaneous.