Answer:
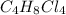
Explanations:
Given the following compounds from the experimental data:
Carbon = 37.5%
Hydrogen = 6.3%
Chlorine = 55.8%
Convert to mass
Carbon = 37.5g
Hydrogen = 6.3g
Chlorine = 55.8g
Covert the mass to moles
Moles of C = 37.5/12 = 3.125moles
Moles of H = 6.3/1 = 6.3moles
Moles of Cl = 55.8/17 = 3.282moles
Divide by the lowest number of moles
For Carbon: 3.125/3.125 = 1
For Hydrogen: 6.3/3.125 = 2.016
For Chlorine: 3.282/3.125 = 1.05 moles
Determine the empirical formula
CH2Cl
Determine the molecular formula
(CH2Cl)n = 127
(12 + 2 + 17)n = 127
31n = 127
n = 127/31
n = 4.09 = 4
The molecular formula will be (CH2Cl)4 = C4H8Cl4