Answer
1) Charle's law
2) The new volume at -4.0 °C = 1.8 L
Step-by-step explanation
1) Charle's law
2)Given:
Initial volume of the soda bottle, V₁ = 2.0 L
Initial temperature, T₁ = 25.0 °C
Final temperature, T₂ = -4.0 °C.
The Charle's law equation is:
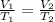
What to find:
The new volume, V₂ at -4.0 °C
Before plugging the values of the given parameters into the equation, the temperature must be converted from °C to K.
T₁ = 25.0 °C = 25 + 273.15 K = 298.15 K
T₂ = -4.0 °C = -4.0 + 273.15 K = 269.15 K
Therefore, to get the new volume, V₂, put V₁ = 2.0 L, T₁ = 298.15 K, and T₂ = 269.15 K:
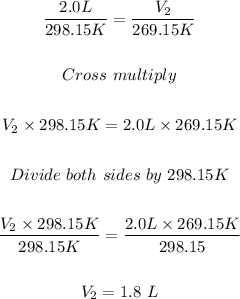
Hence, the new volume, V₂ at -4.0 °C = 1.8 L