ANSWER
All reactants
OPTION D
Explanation:
Firstly, you need to know the meaning of the solution
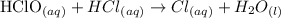
Solution: Solution is defined as a homogenous mixture of two or more components in which particle size is smaller than 1nm.
Solution = solute + solvent
The solute is a substance that is dissolved in the solvent.
An aqueous solution is defined as a solution in which its solvent is water
As you can see from the above-balanced chemical equation, the solutions in the reaction have a subscript (aq) attached to them.
Also, from the reaction, the compounds that are in reactions are the reactants only which are HClO (aq) and HCl (aq)
Hence, the answer is all reactants -------- OPTION D